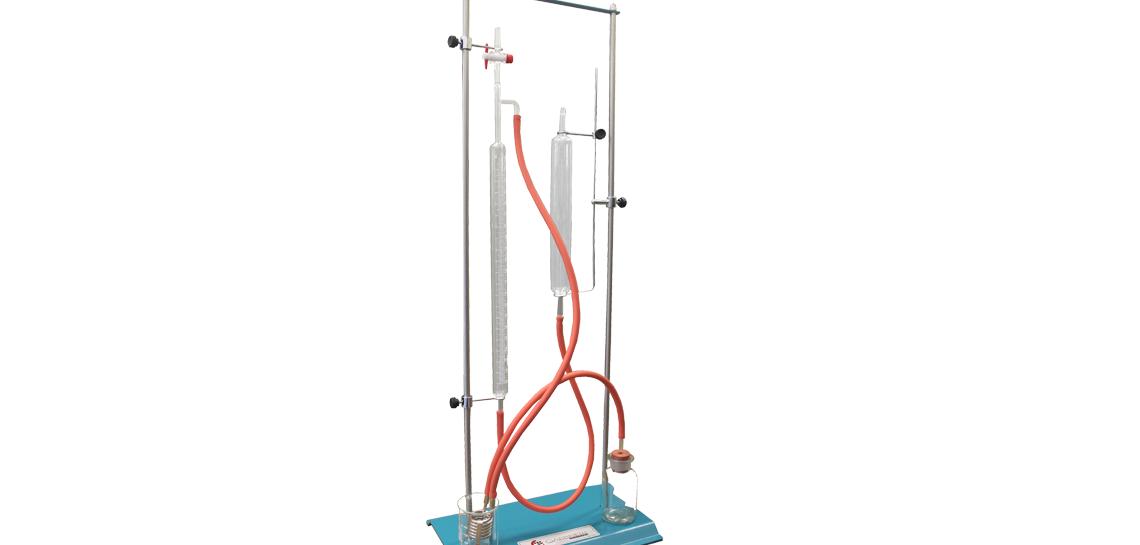
Dietrich-Fruhling Calcimeter
Instrument to determine the fast contents of CaCo3 (calcium carbonate) according to the method Dietrich-Fruhling. Essentially consisting of a sample-holder, one serpentine for cooling and one graduated cylinder with readings on the result of reaction between calcium carbonate and diluted chloridric acid. Since the volume of Co2 (carbonic anhydride) is in relationship with CaCo2 (carbonate contained in the material) it shall be possible to calculate the percentage of CaCo3.
TECHNICAL SPECIFICATIONS
• Steel epoxi painted base
• Chromium plated steel rods
• Bottle for sampling
• Graduated glass test-tube 0 ÷ 200 c
• Collecting glass container for returned water
• Cooling coil
| Code | Model | External dimensions | Weight |
|---|---|---|---|
| GT0156 | DIETRICH-FRUHLING CALIMETER | 400x250x1100 mm | 13 kg |
| Code | Description | Image |
|---|---|---|
| GT0158 | Kit glass parts | |
| GT0159 | Kit metallic parts | |
| GT0160 | Cooling coil |
Related products
Pizzarelli Calcimeter
Description:
Instrument for the rapid % determination of the contents of calcium carbonate (CaCo3) in clays and marnes.
Category:
CalcimetersStandard:
Laboratory:
Raw Materials LabInstrument for the rapid % determination of the contents of calcium carbonate (CaCo3) in clays and marnes.
De Bernard Calcimeter
Description:
Instrument for the rapid % determination of the contents of calcium carbonate (CaCo3) as for DE BERNARD method.
Category:
CalcimetersStandard:
Laboratory:
Raw Materials LabInstrument for the rapid % determination of the contents of calcium carbonate (CaCo3) as for DE BERNARD method.
Scheibler Calcimeter
Description:
The Scheibler Calcimeter is designed on the basis of the volumetric measurement method to determine the percentage of carbonate in the soil. In this method,…
Category:
Chemical-Phisical Properties CalcimetersStandard:
Laboratory:
Raw Materials LabThe Scheibler Calcimeter is designed on the basis of the volumetric measurement method to determine the percentage of carbonate in the soil. In this method, the carbonate in the sample is converted into Co2 gas by adding hydrochloric acid and the resulting gas pressure.